Learn the science behind:

The Science of Color in Fruits and Vegetables
Orange, purple, green, or bright red. You can find fruits and vegetables of just about any color out there. Some of them are very stable. Others disappear easily, or can even change color.
Want to control the color of your fruits and vegetables? Then we’d better introduce you to chlorophyll, carotenoids, flavonoids, and betalains. These groups of molecules are the engine behind most of the color in your produce. Understanding how they behave, will help you control color.
What is color?
Before we dig into specific colors of fruits and vegetables. Let’s have a look at what color even is!
Color is visible light. Light that our eyes can detect. But what exactly is light?
Light is waves
Light is a type of electromagnetic radiation. These are waves that travel through the space around us. A lot of different types of waves exist, light is just one. Most of these waves we can’t see, they are outside of the ‘visible spectrum’. Radio waves, microwaves or X-rays. These are also electromagnetic radiation, we just can’t see them.
Electromagnetic waves can be described by their wavelength. Some wavelengths are very long, they can be kilometers long. Others, are very very short. Visible light falls within those two extremes. The wavelength of visible light is about 380-750nm.
1 nm = 1 nanometer, this is 1/1.000.000.000 of a meter.
Wavelength determines color
Once a wave is within the visible spectrum, we can see it. The specific length of that wave that reaches our eyes determines the color we see. For example, 600nm is orange, 450 nm is blue and 550 nm is what we perceive as green.
In reality, measuring and perceiving color are very complex. It depends on a lot of factors, the lighting conditions, the structure of the surface and much more. It is why measuring color is complicated.
A tomato is red, because it reflects red
So why is a tomato red?
For us to perceive the red color, a red wave of light needs to enter our eyes.
Sunlight may seem white, but, in reality, it is a mixture of waves of different lengths. When this beam falls onto a tomato though, the tomato absorbs a lot of these waves. It only reflects the red wavelengths. It’s this reflection that we see.
Pigments absorb & reflect
In order for specific wavelengths to reach our eye, something within a fruit or vegetable needs to absorb and reflect the right waves. Special molecules do this. They can interact with light and absorb and reflect lights of different wavelengths. Their molecular structure allows them to interact with light in this way.
These molecules are referred to as pigments. Pigments aren’t unique to foods. They’re also used to due clothing, give color to paint, etc. Often you only need a little bit of pigment to give something color.
Four types of plant pigments
In fruits and vegetables almost all colors are caused by just 4 groups, or families, of pigments:
- Chlorophyll (green)
- Carotenoids (yellow, red, orange)
- Flavonoids: anthocyanins + anthoxantins (red, blue, purple)
- Betalains (red, yellow, purple)
A family of pigments again consists of a range of different molecules. But, each molecule in one ‘family’ has a similar basic structure. Let’s have a closer look at each of them.
Chlorophyll – The green of this world
You can see chlorophyll all around you. Grass, leaves, stalks, they’re all green because of chlorophyll. All green plants contain chlorophyll. Chlorophyll is crucial for the survival of most plants. It absorbs the energy of sunlight to drive a process called photosynthesis. During this process water and carbon dioxide react into glucose, a crucial energy source, not just for plants.
Chlorophyll doesn’t absorb all the light of the sun. If it would, it would have been black, no light would come off it. Instead, the green wavelengths aren’t that important for photosynthesis. These green wavelengths are reflected and land in our eyes, making everything green.
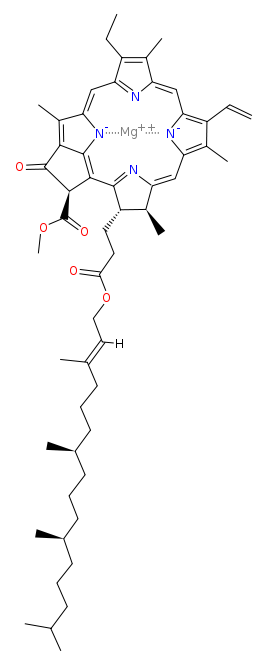
Chlorophyll chemistry
Chlorophyll molecules consist of a long tail with a large ring on top, the heme ring. The ring absorbs light and is responsible for color. The tail just helps the molecule to stay in place and do its job.
When the ring structure breaks down, or gets damaged, the green color will be lost or change in color. This can happen during cooking but also during storage of green fruits and vegetables. A common way for this to happen is contact with heat and acid. The chlorophyll then reacts to become pheophytin, which has a dull green color.
Making a green pistachio ice cream isn’t easy. Chlorophyll isn’t very stable, so it’s hard to keep it bright!
In plants, there are just two types of chlorophyll: a and b. Most plants contain both but in different ratios. The ratio can influence that exact color of the produce since both have a slightly different color profile.

Carotenoids
Does the word ‘carotenoids’ remind you of carrots? If so, that is not a coincidence! Carotenoids were first discovered in carrots, hence the name. However, it’s a large group of different molecules responsible for a lot of red, orange, and yellow colors in fruits and vegetables.
Carotenoids are quite stable molecules. It is why orange carrots stay orange, even after boiling them for some time. But, they aren’t eternally stable either. Exposure to the oxygen in the air can result in some loss of color due to oxidation.
Carotenoids have quite a different structure than chlorophyll. They have long carbon chains with circular structures on the ends.

Types of carotenoids
There are hundreds of different carotenoids. One of the most well-known is β-carotene. You can find β-carotene in carrots, oranges, mangoes, sweet potatoes, and many more fruits and vegetables.
β-carotene doesn’t just provide color. It is also important for human health. Our body can convert β-carotene into vitamin A!
Lutein is another very common carotenoid. You can find it in green leafy vegetables, even though there it’s hidden by the chlorophyll. Zeaxanthin gives color to bell peppers, saffron, and corn. Lycopene is crucial for the color of tomatoes. You can find capsanthin in many pepper species.
Hidden by chlorophyll
A lot of green leafy vegetables contain a lot of carotenoids alongside chlorophyll. Carotenoids often serve to protect chlorophyll and execute several other vital functions. Nevertheless, you can’t actually see these yellow/orange colors. Chlorophyll hides them. Only when the chlorophyll breaks down these colors become visible. It’s the reason why your kale or broccoli may turn yellow in the fridge. The chlorophyll is breaking down, revealing the carotenoids below.
The same applies to a lot of fruits. Unripe, a lot of fruits are green. Only when they ripen will they get their final color. Again, this is because chlorophyll hides the colors. During ripening chlorophyll breaks down and the underlying colors become visible.

Flavonoids
Next up: flavonoids. This is another big group of pigments responsible for the color in many fruits and vegetables. Flavonoids themselves can again be split into two different groups:
- anthocyanins
- anthoxantins
There are hundreds of molecules that belong to each of these classes.
Anthocyanins
Anthocyanins are known for their purple, blue, and red colors. Purple carrots, black raspberries, purple potatoes, they all contain anthocyanins.
Most anthocyanins aren’t very stable. They are very sensitive to the acidity of their environment. They may be a different color altogether under acidic conditions than they are in an alkaline environment.
This can be demonstrated very easily in red cabbage. Red cabbage can be bright pink or dark blue, depending on the acidity of the liquid that surrounds it.


Anthoxantins
Whereas anthocyanins have strong bright colors, its relatives the anthoxantins are almost the opposite. These are white/yellow in color and a lot more stable than anthocyanins. You can find them in cauliflower and onions for instance.
Betalains
Last, but not least, betalains. Betalains aren’t as common as the other three pigment families. They are most common in beets as well as a few other types of produce. They are structurally quite similar to anthocyanins but contain that nitrogen atom that anthocyanins don’t.
Common examples of betalains are betains (these tend to be red) and betaxanthines (these tend to be yellow). Betalains dissolve in water and are sensitive to heat, light, and again pH. It is why cooked beetroots can lose their color during storage. However, they do tend to be more stable than anthocyanins. As such, they are more commonly used to color other foods red.
Note that we humans can’t digest these colors. They just pass through our digestive tract. It is why your urine may be red after you’ve eaten beetroots!


Maintaining & creating color
Cooking, cutting, and just preparing food in general can have a big impact on the color of fruits and vegetables. As we just learned, some colors are quite unstable, whereas others can handle these circumstances somewhat better.
Another aspect that we haven’t discussed yet though, is that new colors may also be formed when preparing food. Some desirable, some less so.
Desirable browning – Maillard
For example, when you grill vegetables, the vegetables will turn brown. This is because of the Maillard reaction. During this reaction brown molecules are formed, but also a lot of very flavorful molecules, changing the color and flavor of your vegetables.
Undesirable browning – Enzymes
On the other hand, especially fruits may be prone to turn an unappetizing brown. After peeling or cutting apples or bananas, they may turn brown rather quickly. This doesn’t necessarily affect flavor, but it does make the fruit a lot less appealing. This color change is due to a process called enzymatic browning.
Now that you understand what makes the color of your fruits and vegetables, it’s time to put it to use. Use your understanding of color to make your fruits and vegetables stand out even more!
Sources
Physics classroom, Light waves and color, link
Physics classroom, Visible light and the eye’s response, link
Wikipedia, Capsanthin, link, visited April-2022
Wikipedia, Zeaxanthin, link, visited April-2022
What's your challenge?
Struggling with your food product or production process? Not sure where to start and what to do? Or are you struggling to find and maintain the right expertise and knowledge in your food business?
That's where I might be able to help. Fill out a quick form to request a 30 minute discovery call so we can discuss your challenges. By the end, you'll know if, and how I might be able to help.





awesome article thanks
thanks for the info