Learn the science behind:

Vanilla vs. Vanillin – Science of Vanilla Flavorings
If you want to give your food a vanilla flavor, you have a range of options to choose from. You can use vanilla vs vanillin, or ethyl vanillin. Synthetic or natural vanilla flavoring. Or, choose between a vanilla extract, a paste, or a bean.
They’re all related, but different. We’ll have a look at their differences and how they evolved to be.
Vanilla flavoring started out with beans
To start our vanilla flavor journey, let’s have a look at the ‘original’: natural vanilla. We humans have enjoyed vanilla flavor for centuries. For instance, we know the Aztecs enjoyed a chocolate drink that was flavored with vanilla. This vanilla was no concoction, or special recipe, instead, vanilla stems from a plant. More specifically, a type of orchid that can only grow in the tropics. This orchid grows pods, that hold its seeds. It’s these pods that, once harvested, can be transformed into vanilla flavor bombs.
Natural vanilla is expensive. An important reason for this is that vanilla orchids can only grow in select regions of the world. They also need a lot of manual labor and attention to thrive.
Processing vanilla beans
A freshly harvested vanilla pod is green and doesn’t yet have a rich vanilla flavor. But, it does contain a lot of precursors. These are molecules that, under the right conditions, can be converted into flavorful components. Through processing the pods, these flavors will come out. Similar to how coffee beans, tea and cocoa need to be processed to properly shine. Processing also causes the vanilla pods, also referred to as vanilla beans, to turn black and shrivel.
Processed vanilla beans contain >200 aroma components!
The resulting black beans are very aromatic. They contain over 200 volatile components. The molecules evaporate easily. They enter your nose where they play a key role in defining flavor.
But, there’s more. Vanilla beans contain sugars, amino acids, fats, and more. All of them contribute to creating a rich, complex vanilla flavor. This is the ‘original’ vanilla flavor.
Using vanilla beans to add flavor
Add the beans to your ice creams, cookies and cake, and you’ll get that characteristic vanilla flavor. You can do so by scraping the seeds from the beans, and adding those in. Alternatively, manufacturers may finely chop the whole bean and add that.
Vanilla extract
To capture the taste, without having to add pieces, you can also opt for using an extract from these beans. You will still get the complex flavor profile.
In a vanilla extract the flavors of the bean are ‘extracted’ into whichever liquid you’re using. This is often a liquid with a high alcohol content, which helps to pull out those flavor molecules.
Extraction takes time, but happens pretty much by itself. By placing the vanilla beans in the liquid, the flavor molecules will slowly seep out and sit in the liquid. After some time, most molecules will have left the beans, and moved into the liquid.
Did you know? You can easily make vanilla extract at home. We made a quick visual guide for you.
Making a good vanilla extract tends to take time. Some extracts need to stand for months, if not years, to fully develop their flavors. An alternative therefore, is to extract the flavor of the bean directly into your product. You may need more vanilla to reach the same strength level, but the flavor profile will be very similar.
When making vanilla ice cream you may be asked to soak a vanilla bean in the milk and cream. By soaking it for some time, the flavor molecules can seep into your milk & cream. This process does take some time!
Vanilla paste
Lastly, you can use vanilla paste, yet another version of a vanilla bean. Formulations differ, but most vanilla pastes are a mix of vanilla extract and ground-up vanilla beans. Some might contain some other ingredients, others don’t. But from a chemical perspective, vanilla paste is a vanilla bean.
What makes vanilla taste like vanilla?
Now that we know what vanilla is and where it comes from, let’s have a look at vanillin.
The discovery of vanillin stems from the mid-/late-19th century. Vanilla has been popular for a long time. Over time, supply couldn’t keep up with the demand and vanilla beans became (too) expensive. Even nowadays, vanilla beans are one of the most expensive spices in the world.
So, we humans decided to look for ways to replicate this desirable flavor, without having to use vanilla beans. To do so, chemists got to work to understand what makes vanilla taste like vanilla.
Vanillin makes vanilla taste like vanilla
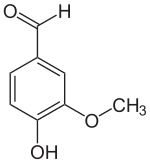
Despite the fact that vanilla beans contain hundreds of flavorful components, chemists found that one molecule in that mix is absolutely crucial for making vanilla taste like vanilla. They appropriately named this molecule: vanillin.
They quickly identified its chemical formula and structure as well. It is quite a simple, small molecule, with the chemical formula C8H8O3. Its chemical name is 4-hydroxy-3-methoxy-benzaldehyde.
The structure of the molecule is similar to that of flavor molecules found in other spices. For example, it shows a lot of similarities with eugenol and cinnamaldehyde. These two molecules are key players in the flavors of cinnamon, cloves, and nutmeg.
Vanillin is a quality indicator for vanilla beans
Since vanillin is a key driver for vanilla flavor, they also found that vanillin content is a good indicator of the strength and quality of the flavor of a vanilla bean. Yes, all the other components do add complexity, but vanillin drives the vanilla flavor.
The vanillin content is heavily influenced by how well a bean was cured and processed after harvest. A good quality bean can contain up to approximately 3% vanillin.
Vanilla beans contain a lot of vanillin, they aren’t the only foods that contain this molecule. Plenty of other spices, as well as fruits, contain small amounts of vanillin. The vanillin serves as just one of many aromatic molecules, building the overall flavor profile.
Artificial vs natural vanilla & vanillin
Remember that vanilla is a complex mixture of a hundreds of components. Together they make up a vanilla flavor. Vanillin is just one molecule in that complex mixture, but an important one.
However, whereas vanilla will always contain vanillin, just because something contains vanillin doesn’t mean it contains vanilla beans. Once the structure of vanillin was discovered, researchers set about to find ways to make vanillin without using vanilla. Over the years several ways to do so have been developed, which we discuss in far greater detail ‘how vanillin is made‘.
Is vanillin artificial or natural?
Whereas you can make vanillin without any vanilla, not all of them are necessarily artificial. Some are made from other natural ingredients, such as the flavor eugenol.
Also, keep in mind that the vanillin in vanilla is natural.
Vanilla beans are always natural
Vanilla beans and its related products such as extracts and paste are always ‘natural’. They’re made directly from the vanilla bean. Some vanillins are natural, others aren’t. This doesn’t just depend on the process that’s used to make them, but also on the legislation in the country you live in.
Ethylvanillin – Vanillin’s artificial powerhouse cousin
There is yet another vanilla flavoring molecule, which is always artificial. This molecule can’t be found in nature and has only been made by humans. It was also discovered in the search for cheaper alternatives to vanilla. Again, it’s just one molecule: ethyl vanillin.
Ethylvanillin has an even stronger vanilla flavor than vanillin, about 2-4x as strong. A lot of artificial vanilla flavorings are made with ethylvanillin. The label might not call out the name.

Which is best? – Vanilla vs. Vanillin
All in all, to give a product that vanilla flavor, you have a lot of options. You can use vanilla beans, an extract, or a paste. Alternatively, you can opt for pure vanillin, or the stronger ethylvanillin. These last two will often be referred to as (artificial) vanilla flavoring, instead of just vanilla.
But, which is ‘best’? Should you use the vanilla bean, an extract, or maybe just the vanillin?
There is no correct answer here. It all depends on personal preferences and what you’re making.
Is vanilla the shining star of a dish? Then you might be interested in that complex, layered flavor. Using a vanilla bean or a high-quality extract can be worth your effort.
Are you just adding a hint of vanilla to a cookie or a cake? In that case, a cheaper (artificial) option may work just as well here*.
Lastly, keep in mind that vanilla beans are a natural product. A poor quality vanilla bean may be a worse choice in some cases, than using pure vanillin.
Overall, it depends on your (and your customer’s) preference! If you’re used to a strong artificial vanilla flavor, you might prefer it over a more delicate, subtle flavor. You might be surprised how big differences between consumers and countries are, despite it being one of the most popular flavors globally!
*Serious Eats did a taste test and found that often differences cannot be tasted in the final product, especially for baked goods!
References
Daphna Havkin-Frenkel, James C. French, Nicoletta M. Graft, Danny M. Joel, Fulya E. Pak, Chaim Frenkel, Interrelation of Curing and Botany in Vanilla (Vanilla planifolia) Bean, Proc. XXVI IHC – Future for Medicinal and Aromatic Plants, Acta Hort. 629, ISHS 200, link
Daphna Havkin-Frenkel, Faith C. Belanger, Handbook of Vanilla Science and Technology, Wiley-Blackwell, 2011, link
V. A. Parthasarathy, Bhageerathy Chempakam, T. John Zachariah, Chemistry of Spices, 2008, chapter 15, link
George A. Burdock, Feranoli’s Handbook of Flavor Ingredients, 2016, 6th edition, p.674, link
What's your challenge?
Struggling with your food product or production process? Not sure where to start and what to do? Or are you struggling to find and maintain the right expertise and knowledge in your food business?
That's where I might be able to help. Fill out a quick form to request a 30 minute discovery call so we can discuss your challenges. By the end, you'll know if, and how I might be able to help.






Ground vanilla powder is very underrated. Scraping the seeds out only gets so much flavor. Dried and ground whole beans offers impressive flavor, especially in baked goods. In a side-by-side test, I found it was way better than extract. You can do it homemade (have a pepper grinder with just vanilla inside it!) or buy it pre-made.
I haven’t tried that yet! Do you find the grinder works well on the vanilla? I tend to have softer/flexible vanilla beans which aren’t that easy to break up.
Thanks for the addition 🙂