Learn the science behind:

How Vanillin is Made
Vanilla beans are expensive and have been so for a long time. So, to satisfy our desire for vanilla-flavored foods, we humans have found a way to make our own vanilla. Or at least, something that tastes like it: vanillin.
In the 19th-century chemists learned that vanillin is responsible for the characteristic vanilla flavor. Before the turn of the century, they also found a way to synthesize it. Nowadays several processes exist, some use wood, other oil, yeasts, or spices to make the coveted molecule.
Vanillin is a core component of vanilla flavor
Vanilla flavor stems from the vanilla bean. This small, but might, bean contains over 200 molecules. Together, they make up the rich, complex flavor of vanilla. However, there is one molecule that really makes vanilla taste like vanilla: vanillin.
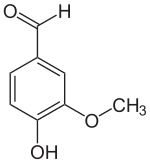
Vanillin’s molecular formula is C8H8O3. Its chemical name is: 4-hydroxy-3-methoxybenzaldehyde. You can buy pure vanillin as a (crystalline), whitish powder. Adding this one ingredient to a food product gives it a distinct vanilla flavor.
Want to learn more about the difference between vanilla vs. vanillin?
Strategies to make vanillin
Vanillin naturally occurs in vanilla beans. But, we can also make vanillin without using a single bean. Over the years, manufacturers have found several ways to do so. Since growing and processing vanilla beans is an expensive endeavour, vanillin made in alternative ways is generally a lot cheaper. What’s more, we currently wouldn’t be able to grow enough vanilla beans to meet the world’s demand for vanilla flavors!
Making vanillin without vanilla bean generally uses one of two processes:
- Transform a similar molecule into vanillin: for this method, manufacturers look for components readily available, that contain a molecule that’s very similar to vanillin. They then isolate the molecule and transform it into vanillin.
- Use a microorganism to make it: ever since scientists found a way to use microorganisms to make insulin (essential for people with diabetes), developments have continued to happen. Manufacturers nonwadays use microorganisms to make rennet, and many other ingredients, including, vanillin!
We’ll have a look at several examples of each.
Natural vs. artifical vanilla flavors
Whether or not these different types of vanillin are artificial or natural, can be rather confusing. Different countries have different sets of legislation.
Generally speaking, if you make vanillin from lookalike molecules that come from the petrochemical industry, the vanillin is artificial. When using microorganisms to make vanillin the discussion becomes a lot more fuzzy. It’s harder to define whether these are natural, nature-identical or artificial and depends on local factors. As such, we won’t be discussing this in any further detail.
Making vanillin from lookalikes
Scientists have found a range of materials that contain molecules which are quite similar to vanillin. These materials need to be abundant and cheap enough to make it worthwhile to harvest these lookalike molecules. Using a series of chemical reactions, they can be converted into vanillin.
A disadvantage of most of these methods is that they require quite harsh chemicals and intense process conditions. Nevertheless, they are commonly used.
Using other spices: Eugenol
Eugenol is a small aromatic molecule, just like vanillin. It can be extracted from spices such as cinnamon, nutmeg, and cloves. Its structure is very similar to that of vanillin, see below. Both have a central benzene ring with the same groups attached to two neighboring carbon atoms: OH- and -OCH3. The 3rd group is different though. Vanillin has an -CHO group, whereas eugenol has a -C3H5 group.
When converting eugenol into vanillin manufacturers focus on transforming this last group into that of vanillin. The conversion of eugenol into vanillin was one of the first conversions invented. A major disadvantage though is that eugenol, though cheaper than vanilla, is still quite expensive.
Using wood pulp: lignin
Lignin is a major component of wood pulp. Lignin is a large molecule, made up of a lot of smaller phenolic components. These individual building blocks, such as conifery alcohol, are quite similar to vanillin. They again have a benzene ring to which three groups are attached. Two of them, -OH and -OCH3, are the same ones as in vanillin.
Under the right conditions, lignin can be broken down into these individual components. These can then be converted into vanillin.

Even though wood pulp is a major source of lignin, you can use other sources. Researchers found ways to use cow dung for instance!
Noticed that your barrel aged whisky has a hint of vanilla? That sure is possible! The barrel contain lignin and during aging some of this may convert into vanillin!
Using petrochemicals: guaiacol
In the 1970s, in a continuous search for cheaper and more efficiently made vanillin, another route was found. This chemical process uses guaiacol. Again, a molecule with a very similar structure to that of vanillin. The main source of guaiacol is the petrochemical industry.
Using microorganisms to make vanillin
Microorganisms are small organisms that often consist of just one cell. Bacteria and yeasts are microorganisms. Over the years, scientists have become better at controlling and managing these microorganism. So much so that they can be used to make specific molecules, such as vanillin.
Doing so would eliminate the need for the harsh chemical processes that are necessary to convert lookalike molecules.
But vanillin kills microorganisms!
However, it’s not a straight forward road. For one thing, vanillin is an antimicrobial. It’s made by plants to combat microorganisms. As such, it can actually be toxic to microorganisms!
Also, the yield, so the amount of material made in a batch, of these methods is generally quite low. Large vessels of microorganisms are necessary to produce just minor amounts of vanillin.
Converting ferulic acid using yeast
Nevertheless, progress is being made. Researchers have found a way to use yeast to convert ferulic acid, again a lookalike of vanillin, into vanillin. Unfortunately, ferulic acid is also expensive, driving up costs.
Bacteria can convert eugenol
Whereas we can use chemicals to convert eugenol into vanillin, it might also be possible to use bacteria. These bacteria do the same as the chemical process does, but generally in a less harsh way.
Make vanillin from plastic?!
Last but not least, scientists have recently – in 2021 – found a way to use E. coli bacteria to convert plastic into vanillin! They first break down PET plastic into terephthalic acid and some other molecules. The bacteria can then transform this acid into vanillin.
It’s not a fully operational technology just yet. But, it comes to show, that ever since the mid-19th century we keep on craving vanilla – be it in ice cream or cookies. And, we keep on finding new ways to get it. Who know how we get our vanillin in a few decades from now?
Sources
Melody M. Bomgardner, The Problem with Vanilla, C&EN, Sep-14, 2016, link
Simon Cotton, Vanillin, 14-July, 2010; a podcast by Chemistry World (Royal Society of Chemistry), link
Nethaji J. Gallage and Birger Lindberg Møller, Vanillin–Bioconversion and Bioengineering of the Most Popular Plant Flavor and Its De Novo Biosynthesis in the Vanilla Orchid, Molecular Plant 8, 40–57, January 2015, link
Leanna Garfield, Coal is used to make a surprising everyday ingredient in food, May-8, 2016, link
S Wallace and J Sadler, Microbial synthesis of vanillin from waste poly(ethylene terephthalate), Green Chem., 2021, DOI: 10.1039/d1gc00931a
Method of preparing vanillin from eugenol, patent no.: US3544621A, 1967, by: Alberto Fiecchi, Gian Mario Nano, Giorgio Cicognani, link
What's your challenge?
Struggling with your food product or production process? Not sure where to start and what to do? Or are you struggling to find and maintain the right expertise and knowledge in your food business?
That's where I might be able to help. Fill out a quick form to request a 30 minute discovery call so we can discuss your challenges. By the end, you'll know if, and how I might be able to help.








I have an allergy to both Vanillin and Eugenol and recently discovered that asparagus contains both of these compounds.
I am wondering what other vegetable or maybe fruits contain these chemicals.
They make me very poorly.
Hi Margaret,
I must admit that I do not have a full list of fruits and vegetables that contain eugenol. Generally speaking, you can expect to find eugenol in a lot of spices such as cinnamon, cloves and bay leaf. While doing some research I found this introductory article that seems to cover some of the groups you’re referring to. I do not have enough expertise on allergies to vouch for its correctness, but it might provide a starting point in your further research. Good luck!
I was toiling around finding a cheaper method to make synthetic vanillin and fortunately have found one.